Authors:

Harikrishna KR Nair
Professor and Head of the Wound Care Unit.
Department of Internal Medicine, Hospital Kuala Lumpur Malaysia

Azizi Ayob
Biotechnology Incubation Centre
MRANTI Park, Bukit Jalil Kuala Lumpur, Malaysia.

Faris Hazim
Assistant Medical Officer.
Wound Care Unit, Dept of Internal Medicine,
Hospital Kuala Lumpur, Malaysia.
Chronic wounds include, but are not limited to diabetic foot ulcers (DFU), venous leg ulcers (VLU), and pressure ulcers (PU) that do not progress through the healing process in a timely manner. This case series aims to investigate the recovery of chronic wounds treated with Cellmax™, which contains secretory factors, bioactives and bioproteins from mesenchymal stem cell (MSC) secretome, based on a standard debridement procedure for wound healing and application of subcutaneous injection of Cellmax™ as first-line therapy. Seven patients with different underlying comorbidities consented to participate in the case study for varying treatment duration at the Wound Care Unit, Department of Internal Medicine, Hospital Kuala Lumpur, Malaysia.
The inclusion criteria include patients with chronic wounds classified as Class 2 and Class 3 as according to Harikrishna Periwound skin classification (HSPC). One patient out of seven patients, saw no reduction in wound size, whereas all other had reduced the wound size and the formation of granulation tissues were observed in all patients over time from week 1 to week 6 after therapy. Cellmax™ was also found to be highly tolerable and good safety profile among these seven chronic wound patients.
Wounds Asia 2022 | Vol 5 Issue 3 | ©Wounds Asia 2022 | www.woundsasia.com
Key words:
■ Cellmax™
■ Chronic wounds
■ Secretome
■ Stem cells
■ Wound healing
Chronic non-healing wounds are those that fail to progress according to the normal phase of the healing process in an orderly or timely fashion. They can be classified into diabetic foot ulcers (DFU), pressure ulcers (PU), venous leg ulcers (VLU) and arterial ulcers (Nunan et al, 2014). There are various common clinical features shared by each of these wounds including prolonged or excessive inflammation, persistent infections, formation of drug-resistant microbial biofilms, and the inability of dermal and/or epidermal cells to respond to reparative stimuli (Attingeret al, 2006; Woo et al, 2007; Stojadinovic et al, 2008). The wound healing can be affected by various factors including the patient’s age, comorbidities, health condition, and medications as well as the condition of the wound itself including wound size, location, oxygenation level and the presence of infection (Kee et al, 2019).
Chronic wounds impact heavily on those affected in terms of quality of life and can lead to limb amputations, and even death. The burden potentiates with the rising numbers of lifestyle diseases, with diabetes taking the lead. Statistics from the National Health and Morbidity Survey 2019 in Malaysia shows that the prevalence of high blood sugar elevated significantly from 11.2% in 2011 to 18.3% in 2019, and only half of them are diagnosed diabetes (National Health and Morbidity Survey, 2013). There is an expected growth in the number of DFUs as a complication of uncontrolled blood sugar, posing a significant challenge to the healthcare system and economic burden throughout the nation. A systematic review reveals that the management of DFU per patient in Malaysia is costing around MYR 6,000 in public setting and about 23% higher in private setting, averaging to MYR 8500 per patient annually (Nair et al, 2022).
In recent years, mesenchymal stem cell (MSC) and its secretome have been shown to play an important role in wound healing (Ennis and Bartholomew, 2013). This MSC secretome includes secretory factors, bioactives and bioproteins in the form of protein-based growth factors, cytokines, chemokines and extracellular matrix that could be extracted from MSC and produce stable ingredients that affect wound healing (Wu et al, 2016; Xu et al, 2017). Molecules and secretory factors released by the MSC secretome can promote re-epithelialisation and angiogenesis while inhibiting fibrosis and microbial invasion, all of which aid wound healing. Thus, patients with a chronic wound may require a direct delivery of bioactives and bioproteins-derived functional MSCs via subcutaneous application into the affected area and this could achieve wound healing.
Objective
This study aims to establish the clinical benefits of new subcutaneous product, Cellmax™, which contains secretory factors, bioactives, and bioproteins of MSC secretome, on chronic wounds.
Methodology
Patients with hard-to-heal wounds on their lower extremities were selected via randomised sampling in Wound Care Unit, Hospital Kuala Lumpur and they were observed for a period of 4 to 6 weeks. The patients walked-in to the clinic from Monday to Friday (8am-4pm) and were enrolled in this study based on the following.
Inclusion criteria:
- DFU, VLU, postoperative wound or trauma wound based on the Harikrishna Periwound Skin Classification (HSPC) classification (Table 1) Nair et al, 2020)
- Any wound surface area
- Wound age of at least 4 weeks duration
- Able to comply to at least weekly visit to hospital.
Exclusion criteria:
- Ischaemic wounds before effective revascularisation
- Neoplastic wounds
- Underlying abscesses or fasciitis which require incision and drainage
- Underlying osteomyelitis.
All previous dressings were removed from the wounds and any remaining medications were rinsed using sterile water. The necrotic material or slough were removed by standard debridement procedure. The wound beds were dried eventually. For patients that had lower pain tolerance, a local anaesthetic, lidocaine gel 5%, was applied for 10–15 minutes and wiped off.
Table 1 – The 2015 Harikrishna Periwound Skin Classification (HPSC) (Nair, 2018)
| Class | Subtype | Periwound condition |
|---|---|---|
| 0 | Normal | |
| 1 | Fibrous tissue/tissue at risk | |
| 2 | A | Exudate centered with desiccation |
| B | Exudate centered with maceration | |
| C | Exudate centered with allergy | |
| 3 | Inflammation without infection | |
| 4 | Inflammation with infection | |
| 5 | Atypical (senescent cells/cancer/subcutaneous emphysema) |
Preparation method
Freeze dried Cellmax™ in 2ml vial was reconstituted with 2ml sodium chloride solution for a standard dose for all patients. Cellmax™ was highly dissolved with no sediment noted. Cellmax™ was applied by subcutaneous (SC) injection into the wound edge, carried out by a medical personnel in the clinic. This SC injection refers to the application of a short needle injection into the tissue layer between the skin and muscle. The SC injection was applied at different periwound points within 1.0 to 3.0mm deep into wound edge and wound base/floor. The number of SC injection is based on wound area as stated in Table 2. The SC injection site was approximately 0.5cm-1.0cm between each injection and it follows surrounding the wound edge. The final amount of Cellmax™ used was based on the wound size area.
Wound assessment procedure:
During follow-up visits, all patients were assessed as follows:
- The wound bed was rubbed with sterile gauze and any detachable materials were
fully removed. - The length and width of wound bed were measured by using a standardised scale unit. The measurement of wound size/ lesions (length x depth) and their healing progress were assessed every time after the dressing was changed.
- The wound bed and periwound were also assessed on its general condition, and any side effects were analysed weekly until the point of granulation starts.
- Once the physical assessment was completed, the primary and secondary wound dressing were applied as a standard wound care procedure.
Table 2 – Wound size and subcutaneous injection technique
| Wound size area measurement(square/cuboid) |
Amount of Cellmax™ | Number of injection (SC injection session) |
|---|---|---|
| 5cm x 5cm (or 25cm²) |
1 vial mix with 2ml Sodium Chloride (Amount 2ml) |
6–8 SC injection site Approx 0.25ml per injection |
| 7.5cm x 7.5cm (or 56 cm²) |
2 vials mix with 2ml Sodium Chloride (Amount 4ml) |
10–12 SC injection site Approx 0.25ml per injection |
| 10cm x 10cm (or 100cm²) |
3 vials mix with 2ml Sodium Chloride (Amount 6ml) |
14–16 SC injection site Approx 0.25ml per injection |
| More than 10cm x 10cm (or 100cm²) |
4 vials mix with 2ml Sodium Chloride (Amount more than 8ml) |
16–20 SC injection site Approx 0.25ml per injection* (To assess by the Dr) |
| Note: *Recommended dose and number of injection of Cellmax™ | ||
Table 2 – Demographics of patients and wound condition
| Patient | Gender | Age(year) | Type of wound | Duration of wound | Duration of treatment with Cellmax™ |
Wound bed area at baseline (cm²) |
|---|---|---|---|---|---|---|
| 1 | Female | 73 | VLU | 1 year | 7 days | 110.5 |
| 2 | Male | 62 | DFU | 2 years | 42 days | 30.0 |
| 3 | Male | 60 | Trauma | 7 years | 42 days | 126.0 |
| 4 | Male | 63 | VLU | 11 months | 35 days | 17.0 |
| 5 | Male | 64 | DFU | 4 years | 21 days | 41.5 |
| 6 | Male | 83 | DFU | 1 month | 7 days | 7.0 |
| 7 | Male | 73 | DFU | 2 years | 7 days | 43.0 |
| Note: Diabetic foot ulcers (DFU) and venous leg ulcers (VLU). | ||||||
Ethics approval and consent to participate:
The case series were carried out as per the Declaration of Helsinki’s guidelines and approved by the hospital review board.The objective of the study was explained to study participants. Patients gave consent to participate in this study and use clinical images, and case details for publication/research purposes before the start of the study.
All study data were collected by the investigators and the case record forms were kept at the Wound Care Unit, Department of Internal Medicine, Hospital Kuala Lumpur, Malaysia. All investigators involved were certified based on the Malaysian Good Clinical Practice (GCP), and the study implementation and data management were in accordance with the Malaysian Good Clinical Practice.
Case study
Results
We recruited 7 patients in this observational study, with diabetic foot ulcers (n=4), venous leg ulcers (n=2), and postoperative leg ulcer (n=1). Table 2 shows the demographics of the patients and their wound conditions before therapy or at baseline. There is one female and six males age ranged 60–83 years old (median age: 68 years). The wound duration is from 1 month to 7 years.
The case history of all seven patients which include summary of underlying diseases, diagnosis, medications and their wound conditions during follow-up and while on therapy are shown in Case 1–7.

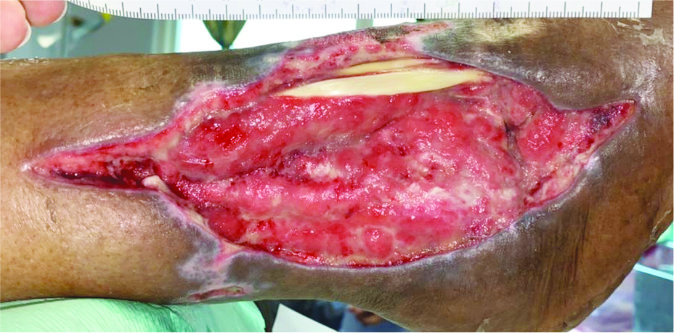
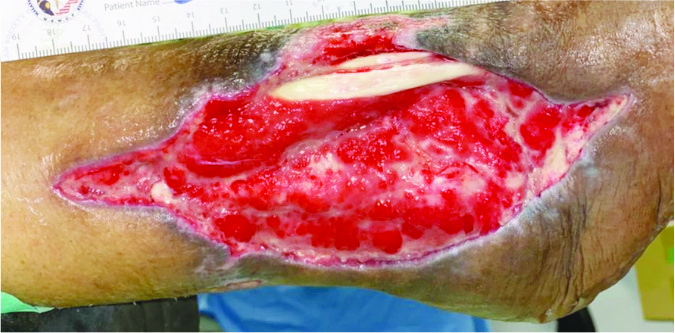
Case 1 – Venous leg ulcer
Case History:
- A 73-year-old female with underlying hypertension, ischaemic heart disease, and Parkinson disease.
- Diagnosis: right venous leg ulcer.
- Medications: amlodipine, perindopril, levodopa, benzhexol, aspirin.
| Before treatment | After 1 week of treatment |
|---|---|
 |
 |
| Wound Size: 110.5cm² Wound bed pale red Normal granulation Creamy yellow tendon |
Wound Size: 110.5cm² Wound bed bright red Normal granulation |
Case 2 – Postoperative on Diabetic charcoat foot
Case History:
- A 62-year-old male, with underlying diabetes, hypertension, ischaemic heart disease, dyslipidemia and chronic kidney disease
- Diagnosis: postoperative on diabetic charcoat foot
- Medications: aspirin, metformin, perindopril, metoprolol, atrovastin, vit b complex and insugen.
| Before treatment | After 6 weeks of treatment |
|---|---|
 |
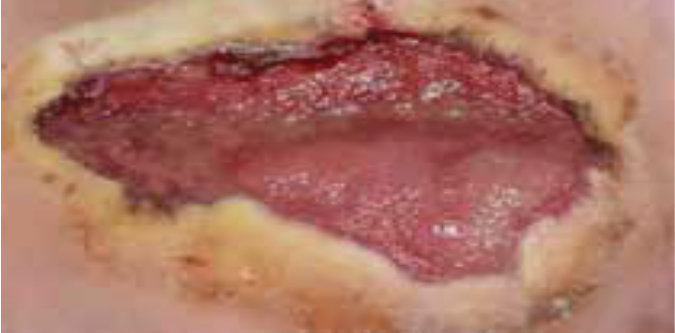 |
| Wound Size: 30.0cm² Wound bed pale red 50% maceration |
Wound Size: 26.4cm² Wound bed pale red 0% maceration |
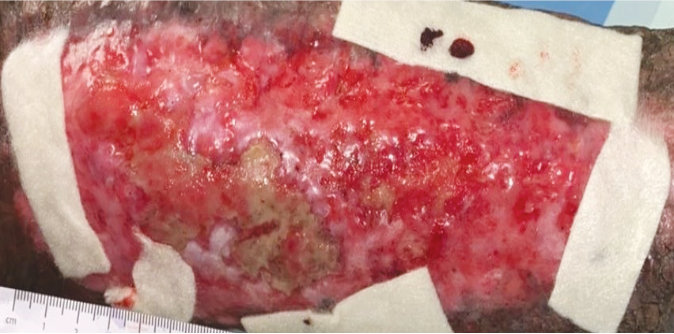
Case 3 – Trauma wound
Case History:
- A 60-year-old male, with underlying diabetes, hypertension, ischaemic heart disease and dyslipidemia
- Diagnosis: trauma wound
- Medications: aspirin, valsartan, bisoprolol, simvastatin, sitagliptin/metformin, gliclazide mr, esomeprazole, ferrous fumarate, folic acid, ascorbic acid.
| Before treatment | After 6 weeks of treatment |
|---|---|
 |
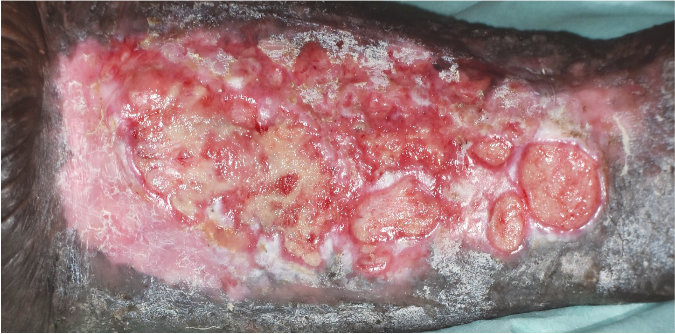 |
| Wound Size: 126.0cm² Wound bed bright red Normal granulation Little epithelization 40% stubborn slough |
Wound Size: 104.5cm² Wound bed bright red Normal granulation 20% epithelization 40% stubborn slough |
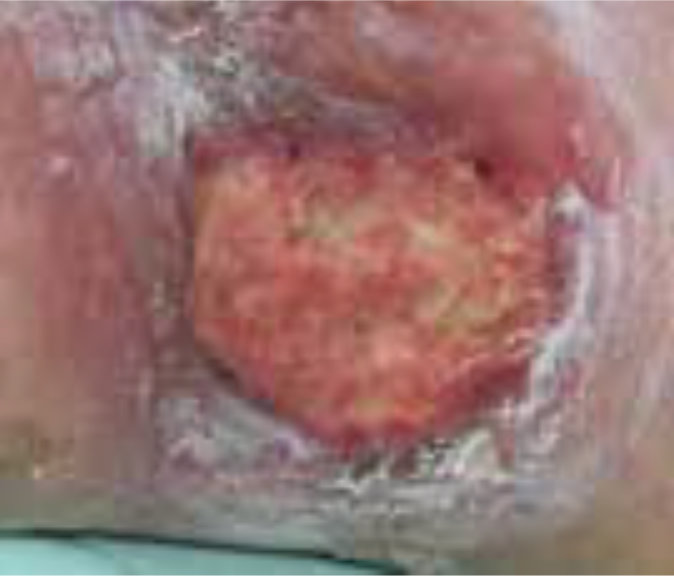
Case 4 – Left Venous leg ulcer
Case History:
- A 63-year-old male with underlying: diabetes, hypertension and dyslipidemia
- Diagnosis: left leg venous ulcer.
| Before treatment | After 3 weeks of treatment |
|---|---|
 |
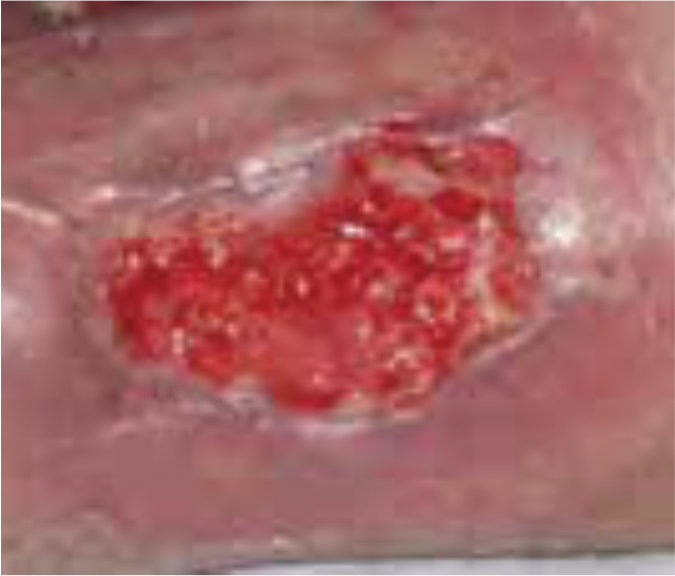 |
| Wound Size: 17.0cm² Wound bed mainly covered with 98% slough Little to no granulation |
Wound Size: 12.1cm² Wound bed bright red 50% granulation 10% slough |
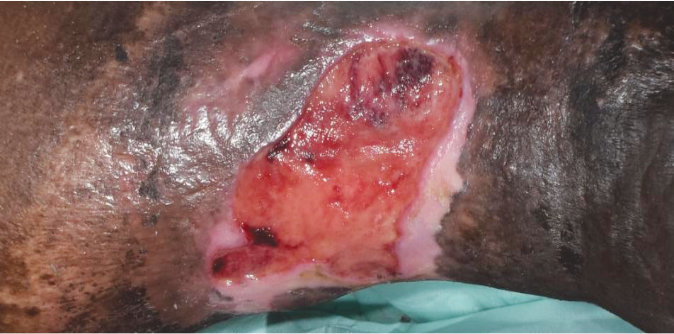
Case 5 – Chronic Venous leg ulcer
Case History:
- A 64-year-old male, with underlying diabetes
- Diagnosis: chronic venous ulcer
| Before treatment | After 2 weeks of treatment |
|---|---|
 |
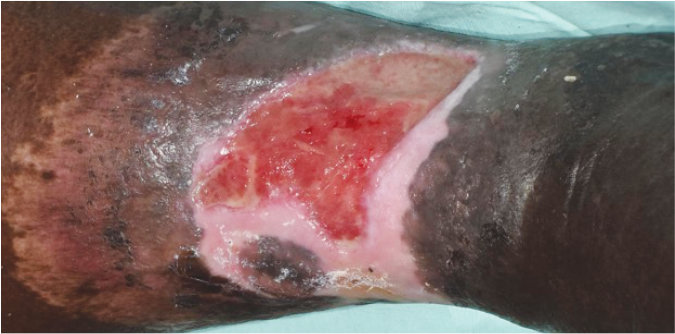 |
| Wound Size: 41.5cm² Wound bed pale red Little to no granulation |
Wound Size: 38.6cm² Wound bed bright red 50% granulation |
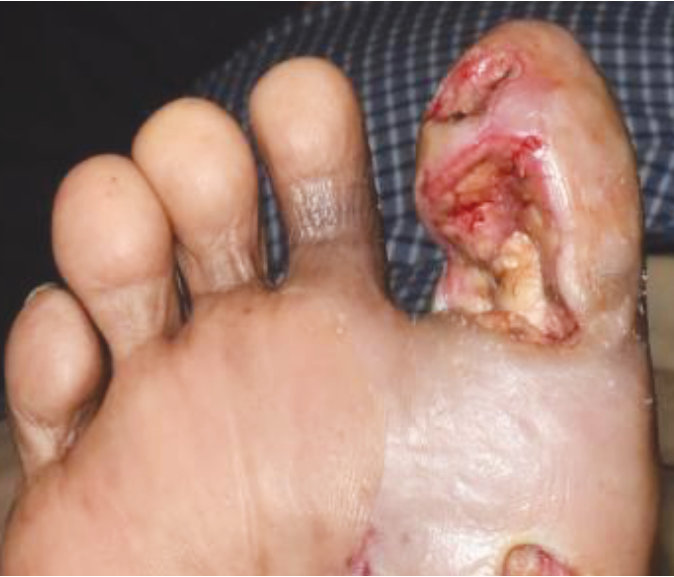
Case 6 – Diabetic foot ulcer
Case History:
- A 83-year-old male with underlying disease: diabetes, hypertension and heart disease
- Diagnosis: diabetic wound (right foot)
| Before treatment | After 1 week of treatment |
|---|---|
 |
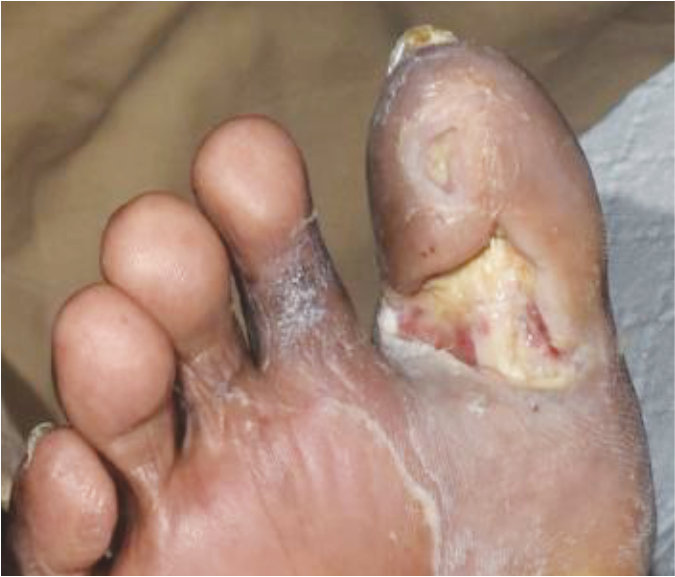 |
| Wound Size: 7.0cm² Wound bed pale red covered with slough |
Wound Size: 4.6cm² Wound bed bright red No slough Granulation noted |
Case 7 – Diabetic foot ulcer
Case History:
- A 73-year-old male, with underlying diabetes, hypetension, heart disease, dyslipidemia and chronic limb ischaemia
- Diagnosis: diabetic wound
- Medications: vastarel XR, coplavix 75/100, amiodarone, frusemide, nebivolol, spironolactone, imdur, entresto, glucophage xr, trajenta, jardiance, enerzair
| Before treatment | After 1 week of treatment |
|---|---|
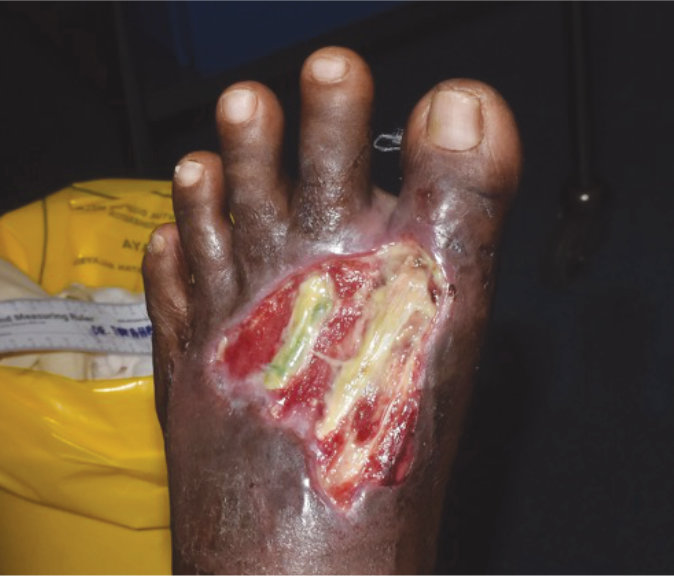 |
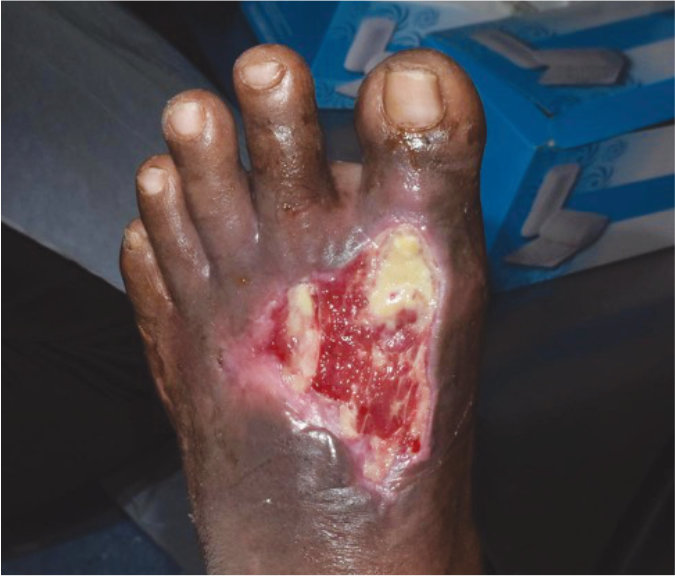 |
| Wound Size: 43.0cm² Wound bed red Normal granulation Little slough |
Wound Size: 41.cm² Wound bed bright red More granulation tissue |
Discussion
Several studies on stem cell-derived secretory factors or also known as the MSC-secretome without stem cells can promote tissue repair when in different conditions (Pawitan et al, 2014). The secretomes contain cell-secreted factors that could be used for regenerative treatment, which include wound healing and it is relatively safe to the human body. However, MSC-secretome can regulate the inflammatory response, stimulate angiogenesis, remodelling of the extracellular matrix, suppress apoptosis, mediate chemoattraction, and affect fibrosis (Deffune et al, 2020).
A study reported by Chen et al (2021) demonstrated that the use of the secretomes to treat wounds in in vivo assays significantly accelerated wound closure and improved regeneration outcome. The study demonstrated that wounds of all groups were almost closed after 15 days, and the secretomes had a significant effect on promoting normal skin regeneration, inhibiting fibrosis and enhancing restoration of the appearance of damaged skin in vivo (Chen et al, 2021). A recent clinical study has demonstrated the wound healing process of a 17-year-old male treated with Wharton’s Jelly-derived MSC-cellular matrix topical gel improved significantly and the lesion had reduced in size (Chandra et al, 2022).
In our study, it was observed only one patient (Case 1) out of seven, saw no reduction in wound size, whereas all other had reduced the wound significantly over time from week 1 to week 6 of therapy. Despite no changes on wound size for Case 1, the wound bed was found to be bright red and normal granulation tissues were noted after 7 days. As the duration and frequency of treatment for the patients recruited differed significantly, and results varied substantially. However, the improvement pattern for all cases indicates that the wound healing rate picks up significantly after more than 7 days of treatment with Cellmax™.
We treated four diabetic foot ulcers with multiple comorbidities with Cellmax™ for between 7 to 42 days, and all of them had a significant reduction in wound size. Their wound beds became bright red, with less slough and more granulation tissue. It was also noted that the previously sloughy tissues had become viable, and the exudate had dried up. Normally, skin wound healing will take around 2 weeks dependent on the wound severity and it will also be affected by existing comorbidities (Szpaderska et al, 2003; Kim et al, 2020). Likewise in our present study, the DFUs age were from 1 month to 2 years. This indicates the severity of the chronic wound condition and complication of poorly controlled blood sugar among the patients. The thwarted wound healing might be related to poor offloading for DFUs and compression therapy for VLUs (Kavitha et al, 2014; Raffettoet al, 2020).
From clinical observation, there is no dermatitis, periwound abrasion, skin infection, skin reaction and localised pain following the subcutaneous injection of Cellmax™, thus indicating good safety profile and suitability of MSC secretome used in the Cellmax™ formulation for the human body. Since the MSC secretomes only consists of cell secreted proteins and bioactives, the used of Cellmax™ for treatment has a very low risk of side effect, and unlikely to have graft versus host disease, and free from infectious disease. Having periwound tissue intact would speed up the wound healing process and leave the patient generally more comfortable during the recovery period. The improvement demonstrated in wound size, excretion of exudate and formation of granulation tissues reflected on patients with different underlying comorbidities, suggesting Cellmax™ works for patients with varying aetiologies.
Limitations:
Limitations for this study include the lack of monitoring over nutrition intake, blood sugar control, length of the treatment period, disease status, and chronic wounds classified as Class 4 and 5 as according to HSPC were not included as more aggressive treatment would be required for such wounds. More patients with a standard follow-up period and therapy regime should be conducted to further access the case studies.
Declaration of interest:
Dedikasi ABA Biosciences supplied the Cellmax™ used for this study. However, the authors have no conflicts of interest to declare.
Conclusion:
This case series demonstrated preliminary clinical efficacy of Cellmax™ containing secretory factors, bioactives and bioproteins in treating chronic wounds by promoting wound closure and improved regeneration outcome. Significant wound size reduction, exudates drying, and formation of granulation tissues indicate that Cellmax™ works for patients with DFUs, venous and arterial lower limbs ulcers. The highly selective action on wound tissue and simple application of this SC injection shows its potential to be a valuable insert for local wound care management in the healthcare setting and outpatient treatment. Cellmax™ was also found to be tolerable and had a good safety profile among seven chronic wound patients.
Attinger CE, Janis JE, Steinberg J et al (2006) Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg117:72S–109S. https://doi.org/10.1097/01. prs.0000225470.42514.8f
Chandra CC, Pratiwi YI and Tan ST (2022) Potential application of Wharton’s jelly-derived mesenchymal stem cells conditioned medium (WJMSCs-CM) on delayed wound healing: a case report. J Pharm Res Int 2022; 34:1–6. https://doi.org/10.9734/jpri/2022/ v34i4A35393
Chen L, Cheng L, Wangg Z, et al. Conditioned medium- electrospun fiber biomaterials for skin regeneration. Bioact Mater 2021; 6:361-374.
Deffune E, Prudenciatti A, Moroz A (2020) Mesenchymal stem cell (MSC) secretome: a possible therapeutic strategy for intensive-care COVID-19 patients. Med Hypotheses142:109769. https://doi.org/10.1016%2Fj. mehy.2020.109769
Ennis WJ, Sui A, Bartholomew A (2013) Stem cells and healing: impact on inflammation. Adv Wound Care (New Rochelle) 2(7):369–378. https://doi. org/10.1089%2Fwound.2013.0449
Kavitha KV, Tiwari S, Purandare VB et al (2014) Choice of wound care in diabetic foot ulcer: A practical approach. World J Diabetes 5(4):546-56. https://doi. org/10.4239%2Fwjd.v5.i4.546
Kee KK, Nair HKR, Yuen NP (2019) Risk factor analysis on the healing time and infection rate of diabetic foot ulcers in a referral wound care clinic. Journal of Wound Care 28(Sup1): S4–13. https://doi. org/10.12968/jowc.2019.28.sup1.s4
Kim HS, Chen J, Wu LP et al (2020) Prevention of excessive scar formation using nanofibrous meshes made of biodegradable elastomer poly(3-hydroxybutyrate-co-3hydroxyvalerate). J Tissue Eng 11: 2041731420949332. https://doi. org/10.1177/2041731420949332
Nair HKR, Chong SS, Othman AM (2020) Validation of Harikrishna Periwound Skin Classification for wound assessment. J Wound Care 29(Sup4):S44–8. https://doi. org/10.12968/jowc.2020.29.sup4.s44
Nair HKR, Norlizah P, Mariam MNet al (2022) Diabetic foot ulcer in Malaysia: consensus on treatment patterns, health care utilization and cost. [Online ahead of print]. Int J Low Extrem Wounds https://doi. org/10.1177/15347346221090096
National Health and Morbidity Survey 2019 Ministry of Health Malaysia. https://tinyurl.com/yc88dy62 (accessed 10 October 2022)
Nunan R, Harding KG, Martin P (2014) Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech 7(11):1205–13. https://doi.org/10.1242/ dmm.016782
Pawitan JA (2014) Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int 2014:965849. https://doi.org/10.1155/2014/965849
Raffetto JD, Ligi D, Maniscalco R et al (2020) Why
venous leg ulcers have difficulty healing: overview
on pathophysiology, clinical consequences, and treatment. J Clin Med10(1):29. https://doi.org/10.3390/ jcm10010029
Stojadinovic A, Carlson JW, Schultz GS et al (2008) Topical advances in wound care. Gynecol Oncol 111(2 Suppl):S70–S80. (2 Suppl)
Szpaderska AM, Egozi EI, Gamelli RL et al (2003) The effect of thrombocytopenia on dermal wound healing. J Investig Dermatol 120(6):1130–7. https://doi. org/10.1046/j.1523-1747.2003.12253.x
Woo K, Ayello EA, Sibbald RG (2007) The edge effect: current therapeutic options to advance the wound edge. Adv Skin Wound Care 20:99–117. https://doi. org/10.1097/00129334-200702000-00009
Wu J, Zhu J, He C et al (2016) Comparative study of heparinpoloxamer hydrogel modifified bFGF and aFGF for in vivo wound healing efficiency. ACS Appl Mater Interfaces. 8(29):18710–21. https://doi. org/10.1021/acsami.6b06047
Xu H-L, Chen PP, ZhuGe DL et al (2017) Liposomes
with silk fifibroin hydrogel core to stabilize bFGF
and promote the wound healing of mice with deep second-degree scald. Adv Healthc Mater 6(19). https:// doi.org/10.1002/adhm.201700344
Wounds Asia 2022 | Vol 5 Issue 3 | ©Wounds Asia 2022 | www.woundsasia.com
